FFR Intas: newffr.intaspharma.co.in/intasffr complete Overview
FFR Intas
Dear readers, I am so grateful for you! Ahmedabad is the home of an international pharmaceutical corporation Intas Pharmaceuticals Limited. It is a manufacturer of generic therapeutic drugs, and conducts studies in clinical development and production in contract. There are 18 facilities for manufacturing including 15 located in India and seven more in both the UK in the UK and Mexico. 31 percent of revenue in the year of 2019 was derived from India and 69% of the revenue came from foreign markets. FFR Intas entire details are available on this website! You can log on to FFR Intas with newffr.intaspharma.co.in/intasffr!
What is Intas Pharmaceuticals Limited?
Hasmukh Chudgar, a Jain pharmacist, started the business in 1977. The company was officially incorporated in the year 1985. The year 2013 was the time ChrysCapital acquired a 16.14 percentage stake in the business and in the year 2015 it sold 10.13 percent of the portion to Temasek Holdings in Singapore; and in 2017 it further reduced the stake it held by selling 3.01 percent in the form of Capital International. ChrysCapital did however buy back the Capital International stake by May 2020. The Chudgar family owns 83.85 percent of the shares owned by the company, and Temasek Holdings and ChrysCapital each have 10.13 percent and 6.02 percent, respectively.
The company has 19 manufacturing facilities in operation in March 2021. 13 of which were located in India five in the UK and one in Mexico. In Bavla close to Ahmedabad the company has built an all-new manufacturing facility which can produce as well as export over 1 billion doses of solid as well as 5 million injectables.
A Brief History of the FFR Intas
FFR Intas is a major global drug definition and advancement manufacturing and marketing firm with global reach. FFR Intas is committed to exploring undervalued clinical and cultural demands through a worldwide value chain for drugs.
- FFR Intas has established an additional organization called Accord Healthcare that will conduct business across the world’s business sector. Intas is growing year on year, through organic growth and acquisitions and expansion of its product range and business operations.
- Intas its outstanding performance on North American and European projects has helped create the brand as a globally recognized brand name.
- Every one the FFR Intas’ products and services reflect the firm’s strict high-quality standards, which can be seen in every single thing FFR Intas is involved in.
- Intas its fragment-driven pipeline as well as the ability to innovate internally allows it to compete against some of the best portfolios of industry. Intas is now focusing its efforts on harder-to-approve modifications, such as Biosimilars or New Chemical Entities, to improve the quality of medical excellence in the shortest time feasible.
- FFR Intas places a high importance on the capacity to make decisions. In the end, Intas is determined to acquire and maintain the highest quality capabilities across the company to make the most efficient use of the various business areas.
Biologics Unit (formerly Intas Biopharmaceuticals)
Hematologist Urmish Chudgar founded Intas Biopharmaceuticals in 2006 as an independent biotechnology department of Intas Pharmaceuticals. The subsidiary and Intas Pharmaceuticals Limited parent, Intas Pharmaceuticals Limited, joined in 2012-2013. The division is responsible for the development and production of biosimilar drugs using monoclonal antibody or recombinant DNA.
The company was subject to the EU-GMP (Good Manufacturing Practices) audit in December 2006 to seek approval for a clinical trial for the biosimilar Filgrastim within Europe. On April 7, 2007, the business was certified as being EU-GMP compliant and was the first business in India to be certified as such. The company introduced Filgrastim its first biosimilar product to Europe as of 2015, to help patients suffering from advanced HIV infection as well as diseases of the immune system, such as neutropenia.
It is also the MCC South Africa has also granted approval to the company’s manufacturing facility and its products that are manufactured by for the Gulf Cooperation Council (GCC), Syria, Yemen, Belarus as well as other health authorities in the country, as well as having been certified by the EU-GMP.
Import Alert
On June 1st, Intas Pharmaceuticals received notification from the US Food and Drug Administration (FDA) that its manufacturing facility in Ahmedabad – a Special Economic Zone (Pharmez) near Matoda had been placed on an ‘Import Alert.’
Import Alerts inform FDA’s field staff as well as people in general that the agency has enough evidence to warrant the detention of goods which appear to be in violation of FDA’s regulations and laws without a physical exam. Products that could be illegal are prohibited banned from distribution within the United States.
The US FDA
The US FDA inspected this facility between November and December 2022 and the business was given an 11-observation form 483, the majority of the inspections focused on quality and integrity of data compliance. “…We found a truck filled with transparent plastic bags with papers that had been shredded and black plastic bags that mostly contained documents that were shredded randomly by hand, mixed along with various scraps of materials” in the FDA’s report on compliance records. In the wake of receiving the form 483 observations, Intas declares it took an immediate, voluntary decision to suspend production and distribution of the products made by the SEZ facility that is designated to US customers. United States.
After the completion of all tests and verifications following the completion of necessary tests and verification, after the necessary testing and verification, US FDA approved Intas to continue to supply 24 essential products that are that are in short supply within the US. In accordance with the USFDA list of critical medicines, the ones are not permitted to use injectable medicines in therapeutic fields such as vitamins and oncology, bacterial infections and cardiology, as well as sedation tablet drugs used in Neurotherapy kidney stone diseases chemotherapy, hormone issues, and heart problems.
Written Statement
Intas stated in a letter in a statement to Forbes India that patient safety will always be its highest priority. Intas has completed a baseline assessment of its quality control systems as well as its personnel, and is now making changes as part of this commitment. They are working in conjunction with experts from outside cGMP. They are also working with the FDA on a strategy to resume manufacturing in the SEZ to be used in their US market, beginning with a couple of key cancer products. They are fully determined to continue its tradition of providing top-quality drugs. The US market is responsible for 18 percent of the company’s total revenue. This, at the time of its March 2022 launch, is estimated at Rs18,400 crore. The company’s domestic operations generate 32% of its total.
Indian Pharmaceutical
The problem goes beyond Intas and includes a number of large Indian pharmaceutical companies who are in a dispute over regulatory issues in a dispute with US FDA. What is the reason they continue to happen? “Due to the increased competition and the increasing bargaining power of buyers prices are rising however, prices are decreasing. The companies have tried to expand the number of their products in order to offset the decline in value, making the task of ensuring compliance with regulatory requirements more challenging, according to Vishal Manchanda who is the senior vice-president of research for Systematix Group. Some that makes up the Indian pharmaceutical industry is speculating about the likelihood that US Big Pharma lobbying against Indian generic producers could result in an increase in regulatory scrutiny.
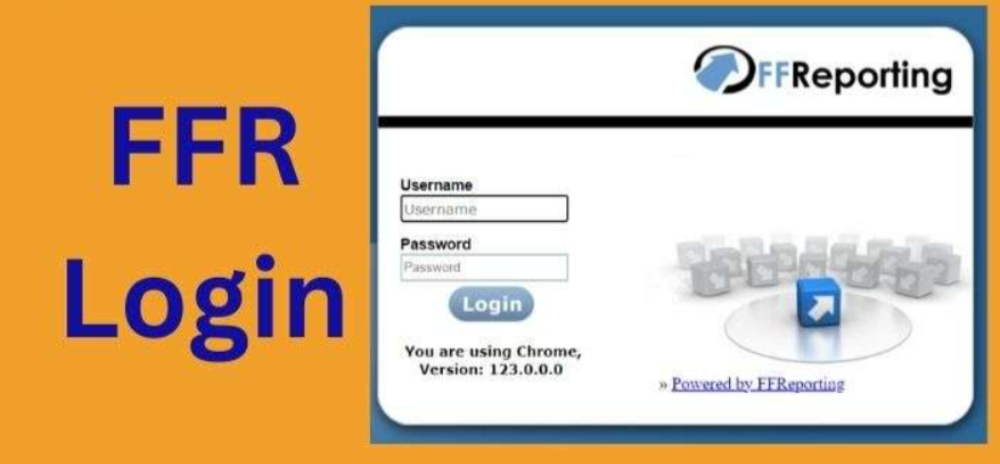
newffr.intaspharma.com/intasffr how to log in?
It’s easy and secure to make use of newffr.intaspharma! To sign in, type the newffr.intaspharma login username as well as password in those fields that are appropriate on your login page. If you lose your password or username it is possible to retrieve your username or password by clicking on the forgotten username, or reset password buttons in the page for login.
When you’ve entered the newffr.intaspharma login details Click”Log In “Log In” button to gain access to newffr.intaspharma and start managing your account. To ensure your security, newffr.intaspharma may request that you enter a one-time number for your mobile number that you have registered.
newffr.intaspharma Important Links
| FFORCE Login – Intas Pharma | https://newffr.intaspharma.com/IntasFFR |
| FForce EOBS – Intas Pharma | https://newffr.intaspharma.com/OnlineEOBS/frmEOBSUpload.aspx |
| FFR Login | https://pharmedltd.in/PLBffr |
| Login | https://intasintraweb.intaspharma.com |
| Newffr.intaspharma.com Catalogs | Daily Catalog | https://daily-catalog.com/newffr.intaspharma.com |
Intas is a world-wide pharmaceutical company. They are fully integrated. Intas concentrates on the research and development of new products. development. “newffr.intaspharma” is their new online platform, which allows users to find out about the latest Intas products including services, medicines, and other products.
By entering the username as well as password in the respective areas on the page to login will enable you to log in newffr.intaspharma.
Other Domain Links
- ffr intas login
- newffr intaspharma
- intas ffr login
- Newffr.intaspharma
- Newffr
- newffr.intaspharma.com/intasffr
- new intas ffr
- ffr login intas
- newffr.intaspharma.com/intas ffr
- newffr.intaspharma.co.in/intasffr
Common Questions
How do I procedure to log in to FFr Intas System?
The first step to log onto the FFr Intas pharmacy system is to start the web browser. Go into the log-in page. Log in using your password and username which was provided via Intas Pharma. After logging in, users will be able to login to Ffr. Ffr system.
How do Reset my FFr Intas pharmacy login credentials to Intas Pharma?
Get support by Intas Pharma’s IT department to reset your FFr Intas pharmacy login credentials by contact them.
How many security precautions are being implemented to protect the FFr Intas pharmacy Login System?
A variety of security measures are put in place to protect security measures for FFr Intas pharma login system. Two-factor authentication is one of them, as are strict requirements for passwords, and periodic security audits. Safe servers have the system also being hosted on. It has the latest security protocols and firewalls.
Does anyone have an app for mobile devices that works with the FFr Intas pharmacy log-in system?
There isn’t any mobile application for login to the FFr Intas pharma login system currently.
Is newffr.intaspharma Secure?
It is true that newffr.intaspharma can be described as a safe platform that comes with additional security features, like authenticating codes that are for registered mobile numbers.
To protect your security and security, newffr.intaspharma recommends that you secure all of your newffr.intaspharma passwords and keep them updated them on a regular basis.
Forgotten Username and Password Link
In the newffr.intaspharma account page you will find useful links such as “forgot username” and “reset password.” These links can help you find your username or password in case you have forgotten your password.
Authentication Code
It is also possible that you will be asked to enter a unique code that is sent to the mobile phone number you have registered by newffr.intaspharma. It’s another security measure.
Customer Support
Customer support on newffr.intaspharma is available 24/7 to help you have any queries or queries about the site.
Conclusion
This blog post provides you with an knowledge of newffr.intaspharma login and the crucial login hyperlinks. You can quickly and securely gain access to newffr.intaspharma as well as manage account information for your FFr Intas account by following these easy steps.